
4 However, the specific cutoff value is laboratory dependent, and thus screen positive and screen negative results are specified on reports. Cutoff levels for ONTD screening are 2.0–2.5 MoM in singleton pregnancies and 4.0–5.0 MoM in twin pregnancies. These factors are used to adjust the multiples of the median (MoM) level. The test is most accurate if the laboratory is also informed of maternal weight, race (white or Black/African American), presence of insulin dependent diabetes, number of fetuses, and family history of ONTD. Ultrasound dating of the pregnancy reduces the false-positive rate and increases the detection rate of ONTDs. 4 If the sample was drawn at <15 weeks, then a new sample should be sent with the accurate gestational age. If there is a discrepancy in gestational age of >10 days after an ultrasound examination, then the test result must be reinterpreted. The laboratory should be informed of the gestational age at the time the sample was drawn for an accurate interpretation. Although NTD screening is optional, pregnancy management is altered by the identification of a fetal neural tube defect independent of the patient’s decision to carry or discontinue her pregnancy. The optimal time for NTD screening is 16–18 weeks’ gestation, but screening can be done between 15 and 20 weeks. 2 MSAFP screening may also detect 85% of ventral wall defects. In the 1980s, maternal serum screening programs became available to identify pregnancies at risk for ONTDs and anencephaly 75–90% of ONTDs and ≥95% of anencephaly can be detected by elevated maternal serum alphafetoprotein (MSAFP) levels with a screen positive rate of 5% or less. 1 This current guideline supercedes the American College of Medical Genetics (ACMG) policy statements entitled Second Trimester Maternal Serum Screening for Fetal ONTDs and Aneuploidy (2004) and First Trimester Diagnosis and Screening for Fetal Aneuploidy (2008) and complements the sections of ACMG’s Standards and Guidelines for Clinical Genetics Laboratories entitled Prenatal Screening for ONTDs and Prenatal Screening for Down syndrome (DS) ( ).

Further, maternal age 35 is no longer recommended as a cutoff for determining which patients should be offered invasive prenatal diagnostic testing.

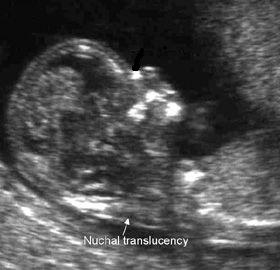
More recently, first trimester screening tests offer women the opportunity of early screening for fetal aneuploidy and the option of earlier diagnosis. Maternal serum screening in the second trimester has now been available for over two decades. A number of screening strategies are available, which utilize both biochemical and ultrasonography to achieve high detection rates. Screening tests to identify women at risk for fetal aneuploidy such as trisomies 21 and 18, and fetal open neural tube defects (ONTDs) are routinely offered to all women during pregnancy regardless of maternal age.


 0 kommentar(er)
0 kommentar(er)
